Tox
Why You Need QWBA for Human Radiolabeled ADME Studies
- Regulatory Guidance
- October 4, 2019
- Satoshi Ito
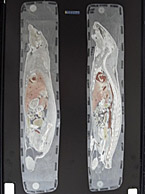
A quantitative whole body autoradiography (QWBA) study provides data required for Human Radiolabeled ADME Studies1. Quantitative whole body autoradiography (QWBA) is an in...
How to Choose the Right Test Systems for Your DMPK Studies
- Test Systems & Methods
- September 12, 2019
- Dr. Chris Bohl, Madison Esely-Kohlman
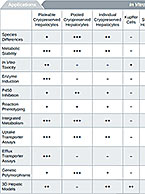
Test systems for DMPK in vitro studies are part of the very foundation of our company. Our labs were borne of...
In Vitro Induction Studies: Elements of Design and Important Considerations in Data Analysis
- Enzyme Induction
- May 13, 2019
- Madison Esely-Kohlman, Dr. Joanna Barbara, Rebecca Campbell
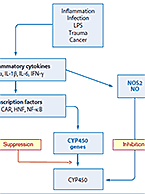
Why do induction studies? Induction potential is an important piece of the drug-drug interaction (DDI) component of an IND submission. Simply put, we...
Minding Your Binding: Plasma Protein Binding Potential Study Now Available at Our US Labs
- Test Systems & Methods
- April 15, 2019
- Madison Esely-Kohlman, Andrea Wolff, Dr. Joanna Barbara
While liver and intestine are important in ADME, behavior in blood is also crucial. When an orally administered drug or...
Studies in Japan: Easier & More Beneficial than You Might Think
- In Vivo & Radiolabeling
- April 8, 2019
- Madison Esely-Kohlman
XenoTech is well-known to pharmaceutical companies and toxicology academics alike for unparalleled experience in quality in vitro ADME/DMPK/DDI studies and complementary products,...
Can Interactions Between Therapeutic Proteins and Small Molecule Drugs Be Evaluated In Vitro?
- Biologics & Oligonucleotides
- April 4, 2019
- Greg Loewen, Dr. Maciej Czerwinski
The mechanisms of clearance for small molecule drugs and therapeutic proteins are fundamentally distinct and therefore therapeutic proteins are generally not...
Important Considerations for the Conduct of In Vitro Drug Transporter Assays
- Drug Transporters
- January 11, 2019
- Greg Loewen, Andrea Wolff, Michael Millhollen
Not only can drug transporters affect the absorption and excretion of drugs, they can be involved in pharmacokinetic-based drug-drug interactions...
To GLP or not to GLP?
- Regulatory Guidance
- October 5, 2018
- Scott Hickman, Dr. Brian Ogilvie, Tim Patterson
That is the question. . . Knowing the answer may save you time and money Good Laboratory Practices (GLP) are...
Choosing a Relevant Small Animal Model for Pharmacokinetic or Toxicity Studies
- Test Systems & Methods
- March 1, 2018
- Dr. Chris Bohl
Choosing the most suitable small animal model is crucial for pharmacokinetic and/or toxicology studies in order to get usable, relevant data. Due to potential...
Subscribe to our Newsletter
Stay up to date with our news, events and research

Do you have a question or a request for upcoming blog content?
We love to get your feedback