In Vivo & Radiolabeling
Toxicokinetic (TK) Analysis for Preclinical Drug Development
- In Vivo & Radiolabeling
- August 6, 2020
- Madison Esely-Kohlman, Jolanta Golec, Dr. Pallavi Limaye
The main goal of preclinical toxicokinetic (TK) studies is to establish a correlation between a candidate compound’s concentration or dose...
Missing Out on Annual ‘Comforting of the Soul’
- Our Team
- May 29, 2020
- Deja Coffin, Madison Esely-Kohlman, Jolanta Golec
As we begin this summer, many of us are grieving opportunities lost for parties and pool days with friends and...
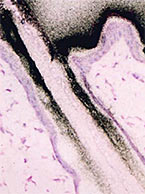
What You Need to Know About Micro-Autoradiography (mARG) Distribution Studies
- In Vivo & Radiolabeling
- December 19, 2019
- Jolanta Golec, Madison Esely-Kohlman
In vivo determination of drug localization in tissue can be uniquely informative to drug developers investigating distribution within the context of...
DDSC In Vivo ADME Expertise Providing Cost Savings
- Updated Offerings
- November 19, 2019
- Scott Hickman
ADME studies are a vital part of the drug development process, and therefore, deciding where and how to perform these studies...
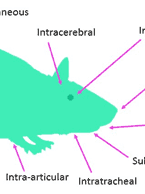
Mass Balance Studies: What You Need and Why You Need It
- In Vivo & Radiolabeling
- November 15, 2019
- Jolanta Golec, Madison Esely-Kohlman
In vivo mass balance studies are an important element of nonclinical drug development, to inform first in-human (FIH) studies and to...
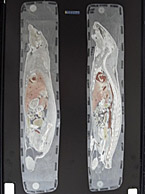
Why You Need QWBA for Human Radiolabeled ADME Studies
- Regulatory Guidance
- October 4, 2019
- Satoshi Ito
A quantitative whole body autoradiography (QWBA) study provides data required for Human Radiolabeled ADME Studies1. Quantitative whole body autoradiography (QWBA) is an in...
In Vivo ADME: What You Need and Why You Need It
- In Vivo & Radiolabeling
- July 22, 2019
- Madison Esely-Kohlman, Satoshi Ito
When putting together a data package for regulatory approval by the FDA, EMA, or PMDA, there is a lot to...
The Story of Us
- Updated Offerings
- July 1, 2019
- Madison Esely-Kohlman, Satoshi Ito
This year at XenoTech we celebrate our 25th birthday, and our partner in Japan turns 64! Looking back over our history,...
Studies in Japan: Easier & More Beneficial than You Might Think
- In Vivo & Radiolabeling
- April 8, 2019
- Madison Esely-Kohlman
XenoTech is well-known to pharmaceutical companies and toxicology academics alike for unparalleled experience in quality in vitro ADME/DMPK/DDI studies and complementary products,...
To GLP or not to GLP?
- Regulatory Guidance
- October 5, 2018
- Scott Hickman, Dr. Brian Ogilvie, Tim Patterson
That is the question. . . Knowing the answer may save you time and money Good Laboratory Practices (GLP) are...
Subscribe to our Newsletter
Stay up to date with our news, events and research

Do you have a question or a request for upcoming blog content?
We love to get your feedback