Drug Metabolism
How can in vitro and in vivo studies help me understand my drug’s clearance?
- Regulatory Guidance
- March 5, 2020
- Madison Esely-Kohlman, Dr. Joanna Barbara
Systemic clearance, denoting how much drug is cleared from a volume of blood per unit of time, is of critical...
Timing of In Vitro Studies: Early, Thorough ADME for Your Compound’s Success
- Regulatory Guidance
- February 13, 2020
- Madison Esely-Kohlman, Dr. Chris Bohl, Greg Loewen
Beyond the need for evidence that a drug works, Food and Drug Administration (FDA) and other regulatory bodies around the...
How Can I Make Sure My Data Meets Regulatory Expectations?
- Regulatory Guidance
- January 10, 2020
- Madison Esely-Kohlman, Greg Loewen
Regulatory authorities publish updated guidance documents that share their expectations for endpoints and test systems with drug developers, but sometimes it is difficult to...
What In Vitro Metabolism and DDI Studies Do I Actually Need?
- Regulatory Guidance
- November 25, 2019
- Madison Esely-Kohlman, Greg Loewen
Though there is no ‘roadmap’ spelling out required studies to achieve regulatory approval for clinical entry, a drug candidate’s metabolism...
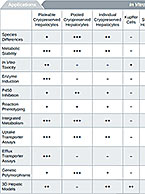
How to Choose the Right Test Systems for Your DMPK Studies
- Test Systems & Methods
- September 12, 2019
- Dr. Chris Bohl, Madison Esely-Kohlman
Test systems for DMPK in vitro studies are part of the very foundation of our company. Our labs were borne of...
Can CYP3A4 Induction Predict P-glycoprotein Induction in DDI Studies?
- Enzyme Induction
- August 20, 2019
- Shanté Jackson, Madison Esely-Kohlman
Generally, a drug’s effects on enzyme and transporter activity are examined independently in a drug development program, but what if...
In Vitro Induction Studies: Elements of Design and Important Considerations in Data Analysis
- Enzyme Induction
- May 13, 2019
- Madison Esely-Kohlman, Dr. Joanna Barbara, Rebecca Campbell
Why do induction studies? Induction potential is an important piece of the drug-drug interaction (DDI) component of an IND submission. Simply put, we...
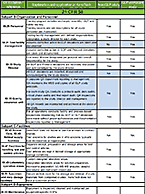
To GLP or not to GLP?
- Regulatory Guidance
- October 5, 2018
- Scott Hickman, Dr. Brian Ogilvie, Tim Patterson
That is the question. . . Knowing the answer may save you time and money Good Laboratory Practices (GLP) are...
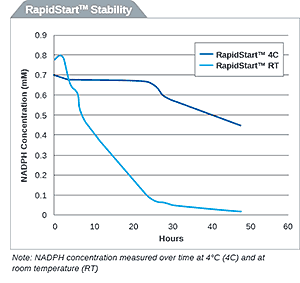
NADPH RapidStart Regeneration System for Extended Metabolism
- Test Systems & Methods
- May 30, 2018
- Isabelle Nobiron
Originally published in tebu-bio’s Being Bioreactive. To purchase XenoTech’s products in the EU, please visit tebu-bio’s website. NADPH is a critical cofactor...
Maximize Metabolic Activities by Limiting Hepatocyte Cryoinjury
- Drug Metabolism
- May 1, 2018
- Michael Millhollen, Dr. Chris Bohl
Cryopreservation and thawing of primary hepatocytes causes inherent damage to the cells. Traditional pooling methods require cells to undergo two separate cryopreservation...
Subscribe to our Newsletter
Stay up to date with our news, events and research

Do you have a question or a request for upcoming blog content?
We love to get your feedback