Author: Michael Millhollen
4 Ways De-Risking Maximizes Compound Value
- ADME 101™
- August 31, 2022
- Michael Millhollen
Since most new drugs fail because of ADME/Tox, you can add considerable value to your compound by conducting early in...
Meet the Scientist: Alex Wakefield
- Meet the Scientist
- February 1, 2022
- Michael Millhollen
In this month’s installment of our Meet the Scientist series we wanted to feature one of our team of talented...
2021 Service Expansions: Microsomal Protein Binding and Red Blood Cell Partitioning
- Updated Offerings
- January 3, 2022
- Michael Millhollen
Over the course of 2021, we expanded access to two important and related study offerings at our US laboratory headquarters....
Meet the Scientists: Program Execution
- Meet the Scientist
- October 27, 2021
- Michael Millhollen
In this installment of our Meet the Scientist series, we asked several members from various areas of our Program Execution...
Meet the Scientists: Analytical Services’ Chandra Kollu & Nadya Galeva
- Meet the Scientist
- September 21, 2021
- Michael Millhollen
This month we’re featuring two of the experts in our analytical services department: Senior Scientist Chandra Kollu and Mass Spectrometry...
ADME/PK & DDI Best Practices Industry Survey Results & Infographic
- Drug Metabolism
- September 12, 2021
- Michael Millhollen
With over 3500 respondents, only 4% of respondents said they had never experienced any repercussions from postponing these studies...
Guide to When & Why to Evaluate ADME/DMPK & Drug-Drug Interactions Available
- Consultancy
- September 12, 2021
- Michael Millhollen
In Safety first: Assessing drugs early can preclude regulatory and health issues, a new ebook developed by a collaboration with...
Why Switch to Hepatocyte Pellets?
- Test Systems & Methods
- June 30, 2021
- Michael Millhollen, Dr. Chris Bohl
You may have heard about the patented CryostaX® format of hepatocyte pellets, but do you know why XenoTech and countless other researchers have made the…
XenoTech Named Most Innovative Drug Development Solutions Provider – USA 2020
- Accomplishments
- August 3, 2020
- Michael Millhollen
The 2020 Technology Innovator Awards returns for its fifth year “to reward those talented and dedicated individuals and firms working...
COVID-19 Updates
- Drug Drug Interactions (DDI)
- March 25, 2020
- Michael Millhollen
As a global Life Sciences and Healthcare company, XenoTech recognizes the seriousness of the Coronavirus Pandemic. We are constantly monitoring...
Meet the Scientist: Andrea Wolff
- Meet the Scientist
- February 20, 2020
- Michael Millhollen
At XenoTech, we pride ourselves on our 3S Principles – the values and standards that guide our businesses and shape...
Meet the Scientist: Mark Horrigan
- Meet the Scientist
- January 27, 2020
- Michael Millhollen
Continuing our effort to put faces to the names of some of the scientists you interact with at XenoTech, we...
XenoTech Named Best In Vitro Drug CRO 2019
- Updated Offerings
- September 19, 2019
- Michael Millhollen
XenoTech has been named the Best In Vitro Drug CRO in the 2019 Healthcare & Pharmaceutical Awards. From the release:...
XenoTech Named a 2019 Top-Performing Provider on Science Exchange
- Updated Offerings
- September 4, 2019
- Michael Millhollen
Science Exchange stated, “Discovery research programs at growing biotech companies are demanding—R&D leaders must balance the need to innovate with...
XenoTech Named Best for Drug Candidate Evaluations 2019, Leading Providers of Pharmaceuticals Safety Testing
- Updated Offerings
- August 12, 2019
- Michael Millhollen
XenoTech has been named the Best for Drug Candidate Evaluations 2019 as well as in the Leading Providers of Pharmaceuticals...
Disease-State Test Systems
- Test Systems & Methods
- January 13, 2019
- Michael Millhollen, Dr. Chris Bohl
XenoTech has been working with and supplying reagents for the pre-clinical ADME field for well over two decades. Years of...
5 Keys to Set Your Contract Research Organization (CRO) Collaboration Up for Success
- Updated Offerings
- January 7, 2019
- Michael Millhollen, Kelsey Acree
Over the past 25 years of performing ADME/DMPK/DDI contract research, we’ve learned some key strategies to maximize the efficiency and effectiveness...
Holiday CSR Activities
- Updated Offerings
- December 26, 2018
- Michael Millhollen, Deja Coffin
XenoTech understands that a better future starts with our actions today, and encourages its global family to remain focused on...
October Presentations on In Vitro Effects of Biologics on CYP Enzymes and Regulatory DDI Guidances
- Regulatory Guidance
- October 26, 2018
- Michael Millhollen
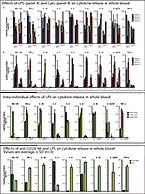
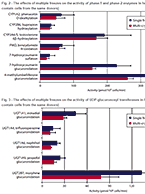
On Oct. 15th at the Peptide ADME Discussion Group Workshop in Gothenburg, Sweden, Dr. Brian Ogilvie presented on In vitro Direct and Cytokine-Mediated...
New Rodent, Monkey CryostaX Hepatocytes Featured in Industry News
- Test Systems & Methods
- September 13, 2018
- Michael Millhollen
Following XenoTech’s announcement XenoTech Adds Monkey, Rodent Hepatocytes to Patented CryostaX Product Line, Anticipates the End of Traditionally Cryopreserved Hepatocytes, various...
Challenges & Solutions in Today’s In Vitro Transporter Research
- Drug Transporters
- August 30, 2018
- Michael Millhollen, Dr. Joanna Barbara
Transporters are membrane-bound proteins that govern the passage of drugs into and out of cells. These gatekeeper proteins can be...
XenoTech Named Best Global CRO 2018; Recognised Leaders in Pharmaceutical Safety Testing 2018
- Updated Offerings
- August 24, 2018
- Michael Millhollen
XenoTech has been named the Best Global CRO and was recognized for leadership in Pharmaceutical Safety Testing in the 2018...
Response to FDA Framework for Assessment of Drug-Drug Interactions for Therapeutic Proteins RFI and Comments
- Regulatory Guidance
- August 6, 2018
- Michael Millhollen
In July, Drs. Maciej Czerwinski, Director of Consulting, and Brian Ogilvie, Vice President of Scientific Consulting, submitted XenoTech’s comments in response to the Food...
No-Cost, On-Demand Primary Human Hepatocyte Pooling Using CryostaX Pellets
- Test Systems & Methods
- June 15, 2018
- Michael Millhollen, Dr. Chris Bohl
CryostaX® hepatocytes are created using a patented process that produces unique single-donor cell pellets. This format allows scientists to easily pool primary...
In Vitro DDI Regulatory Guidance Reference Poster
- Regulatory Guidance
- May 29, 2018
- Michael Millhollen
At the 2018 Marbach Castle Drug-Drug Interaction Workshop in Germany, Dr. Brian Ogilvie, Vice President of Scientific Consulting, presented a comparison...
Maximize Metabolic Activities by Limiting Hepatocyte Cryoinjury
- Drug Metabolism
- May 1, 2018
- Michael Millhollen, Dr. Chris Bohl
Cryopreservation and thawing of primary hepatocytes causes inherent damage to the cells. Traditional pooling methods require cells to undergo two separate cryopreservation...
No More Water Baths! Simplify Hepatocyte Thaws and Eliminate Contamination
- Test Systems & Methods
- April 3, 2018
- Michael Millhollen, Dr. Chris Bohl
XenoTech has patented technology that eliminates the need for the water baths that are traditionally required to thaw cryopreserved hepatocytes. By removing water...
XenoTech Adds US Support for MATE1 and MATE2-K Drug Transporter Studies
- Drug Transporters
- April 2, 2018
- Michael Millhollen
Transporters have become increasingly important in drug development due to the major role they play in absorption, distribution and excretion of...
XenoTech Named Best In Vitro Drug Metabolism Studies Provider 2018
- Accomplishments
- March 27, 2018
- Michael Millhollen
Following our nomination in late 2017, XenoTech has been named the Best In Vitro Drug Metabolism Studies Provider in the...
Insects and Cattle and Sheep, Oh My!
- Test Systems & Methods
- February 15, 2018
- Michael Millhollen, Aaron Hilgedick
Over the past two decades, we’ve received a lot of interesting requests for custom tissue preparations here at XenoTech. “We’ve...
XenoTech Hiring and Growing to Meet Customer Demand
- Updated Offerings
- January 8, 2018
- Michael Millhollen
As announced earlier this month, XenoTech has hired a record number of new staff over the past year in order to...
XenoTech Featured in Fall Issue of NewsWave
- Accomplishments
- November 15, 2017
- Michael Millhollen
Leader in pharmaceutical research and scientific collaboration Originally printed in the Fall 2017 Issue of NewsWave. XenoTech, LLC, which is...
Initial Impressions of New Draft FDA DDI Guidance Documents from XenoTech
- Regulatory Guidance
- October 26, 2017
- Michael Millhollen, Dr. Brian Ogilvie
Updated Nov. 6th, 2017 The FDA has released its long-awaited new draft guidance for industry on drug-drug interaction (DDI) studies....
Further Research on the Drug-Drug Interaction Between Gemfibrozil and Repaglinide Presented
- Drug Drug Interactions (DDI)
- September 27, 2017
- Michael Millhollen
The clinically-relevant drug-drug interaction (DDI) between the dyslipidemia drug gemfibrozil and the antidiabetic repaglinide is well-documented throughout the literature. In...
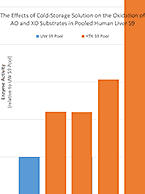
Cold Storage Solution Linked to Lower AO, XO Activity
- Drug Metabolism
- August 22, 2017
- Michael Millhollen
At the time of organ recovery, human livers that have been donated for research are flushed with an ice-cold perfusion...
Considerations In Response to Drug-Drug Interaction Guidances
- Regulatory Guidance
- August 17, 2017
- Michael Millhollen
Check out the recent IQ consortium publication: Considerations from the IQ Induction Working Group in Response to Drug-Drug Interaction Guidances from...
URI Drug Transporters Workshop Presentations
- Drug Transporters
- August 11, 2017
- Michael Millhollen
The University of Rhode Island College of Pharmacy’s 5th Annual Transporters in Drug Discovery and Development: Driving Knowledge from Laboratory...
New Division Directors for Core Services and Logistics
- Updated Offerings
- August 1, 2017
- Michael Millhollen
Following the promotion of Dr. Joanna Barbara, PhD, to Vice President of Scientific Operations, Dr. Etsuko Usuki, PhD, has been promoted...
XenoTech’s Analytical Services Department Adds Instrumentation
- Updated Offerings
- July 17, 2017
- Michael Millhollen
XenoTech’s Analytical Services lab provides researchers with custom method development and method validation, dose solution analysis, in vitro and in vivo metabolite profiling studies, small-molecule non-GLP bioanalysis and...
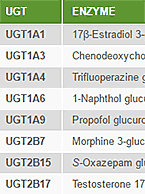
UGT Activities, Concomitant Drugs, and DDI
- Enzyme Induction
- June 20, 2017
- Michael Millhollen
If you have concerns about how your compound may affect UDP-glucuronosyltransferase (UGT) induction and/or inhibition when combined with other therapeutic...
New Liver Disease Resource and Other Research Biobank News
- Updated Offerings
- May 26, 2017
- Michael Millhollen, Dr. Maciej Czerwinski
XenoTech is committed to furthering the knowledge surrounding hepatic diseases, which affects one out of four people worldwide, such as...
Big Hepatocyte News!
- Test Systems & Methods
- May 10, 2017
- Michael Millhollen
As you may have heard, XenoTech was issued U.S. Patent No. 9,642,355 for the “CRYOPRESERVATION OF CELLS AND SUBCELLULAR FRACTIONS” for its CryostaX® hepatocytes. The...
New VP of Scientific Operations and VP of Scientific Consulting
- Consultancy
- May 4, 2017
- Michael Millhollen
XenoTech has appointed Joanna Barbara, Ph.D., as Vice President of Scientific Operations and Brian Ogilvie, Ph.D., as Vice President of Scientific...
XenoTech Adds New Drug Transporters to Portfolio
- Drug Transporters
- April 1, 2017
- Michael Millhollen
Transporters have become increasingly important in drug development due to the major role they play in absorption, distribution and excretion of...
XenoTech Named Best for Pharmaceutical Safety Testing 2017
- Accomplishments
- March 2, 2017
- Michael Millhollen
After our nomination in late 2016, XenoTech has been named the Best for Pharmaceutical Safety Testing in the 2017 Biotechnology...
XenoTech Adds New Dermal Subcellular Fraction Test Systems
- Updated Offerings
- February 1, 2017
- Michael Millhollen
XenoTech is adding human and minipig to the company’s list of species with dermal subcellular fractions available as standard test systems for the development...
XenoTech Featured on Labiotech.EU
- Enzyme Inhibition
- July 5, 2016
- Michael Millhollen
Understand which transporters are involved in a drug’s absorption, distribution & excretion Originally posted on Labiotech.EU How can you build the...
XenoTech Scientists Publish Paper, Present Research Evaluating Ketoconazole and its Alternative Clinical CYP3A4-5 Inhibitors as Inhibitors of Drug Transporters
- Enzyme Inhibition
- April 25, 2016
- Michael Millhollen
XenoTech scientists published a paper in Drug Metabolism and Disposition evaluating Ketoconazole and its alternative clinical CYP3A4-5 inhibitors as inhibitors of drug...
XenoTech Fulfills Largest Order To Date
- Accomplishments
- February 1, 2016
- Michael Millhollen
XenoTech recently fulfilled its largest order of human liver subcellular fractions to date. A large pharmaceutical customer ordered over 30,000...
Are in vitro drug metabolism and drug-drug interaction studies critical for an IND?
- ADME 101™
- November 1, 2022
- Dr. Andrew G. Taylor, Michael Millhollen, Isis Smith, Amara Millhollen
A Guide to What, Why & When to Conduct ADME Studies When drug developers are preparing their IND, drug metabolism...
Spotlight on Efflux and Uptake Drug Transporters in In Vitro Drug-Drug Interaction Studies
- Drug Transporters
- June 23, 2022
- Dr. Andrew G. Taylor, Michael Millhollen, Isis Smith
What are drug transporters? Drug transporters are membrane-bound proteins that assist in the movement of drugs into or out of...
When, Why and How to Conduct CYP2C Induction Studies
- Enzyme Induction
- April 8, 2022
- Dr. Andrew G. Taylor, Rebecca Campbell, Michael Millhollen, Lucy Fahler
Over the years, we have received a lot of questions about cytochrome P450 (CYP) 2C induction studies. In February of...
Which Hepatocytes Should I Use for What Studies?
- Test Systems & Methods
- March 25, 2022
- Dr. Chris Bohl, Michael Millhollen, Isis Smith
Primary hepatocytes are considered the gold standard for ADME/DMPK studies because they are the most representative in vitro test system. However, not all hepatocyte formats…
Why Do Most Polled Researchers Run Red Blood Cell Partitioning Studies with Plasma Protein Binding?
- Drug Drug Interactions (DDI)
- January 25, 2022
- Dr. Steven McGreal, Michael Millhollen
Many compounds bind to or diffuse into red blood cells (RBCs), which can significantly impact clearance and cause inaccuracies in PK calculations...
Interesting Topics at the 3rd SCI-RSC Symposium on Transporters in Drug Discovery and Development
- Drug Transporters
- September 22, 2021
- Andy Rhoades, Dr. Andrew G. Taylor, Michael Millhollen, Cody Hendren
A recap of some of the important and promising research that was presented at the Transporters in Drug Discovery and Development symposium...
Important Considerations for the Conduct of In Vitro Drug Transporter Assays
- Drug Transporters
- January 11, 2019
- Greg Loewen, Andrea Wolff, Michael Millhollen
Not only can drug transporters affect the absorption and excretion of drugs, they can be involved in pharmacokinetic-based drug-drug interactions...
Coverage from the October 2018 PBSS DDI Workshop
- Drug Transporters
- November 5, 2018
- Greg Loewen, Michael Millhollen
The Pharmaceutical and BioScience Society (PBSS) hosted the workshop “Drug-Drug Interactions: Update on Risk Assessment, Clinical Evaluation and Regulatory Requirements” on October...
AAPS-FDA Drug Transporters
- Regulatory Guidance
- May 30, 2018
- Greg Loewen, Michael Millhollen
The American Association of Pharmaceutical Scientists (AAPS) and U.S. Food and Drug Administration (FDA) co-sponsored the workshop “Drug Transporters in ADME:...
XenoTech Featured in Fall Issue of NewsWave
- Accomplishments
- November 15, 2017
- Michael Millhollen
Leader in pharmaceutical research and scientific collaboration Originally printed in the Fall 2017 Issue of NewsWave. XenoTech, LLC, which is...
Considerations When Studying Esterase Activities in the Intestine
- Drug Metabolism
- July 12, 2017
- Dr. Chris Bohl, Michael Millhollen
The gastrointestinal wall is a significant site of first-pass metabolism for oral drugs, which is commonly associated with CYP450 and...
XenoTech Joins Fight Against Liver Disease
- Disease-Specific Resources
- September 16, 2016
- Dr. Maciej Czerwinski, Michael Millhollen
XenoTech is committed to furthering the knowledge surrounding hepatic diseases, such as nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis...
XenoTech Featured on Labiotech.EU
- Enzyme Inhibition
- July 5, 2016
- Michael Millhollen
Understand which transporters are involved in a drug’s absorption, distribution & excretion Originally posted on Labiotech.EU How can you build the...
XenoTech Fulfills Largest Order To Date
- Accomplishments
- February 1, 2016
- Michael Millhollen
XenoTech recently fulfilled its largest order of human liver subcellular fractions to date. A large pharmaceutical customer ordered over 30,000...
XenoTech Hosts Bring Your Kids to Work Day
- Our Team
- May 4, 2015
- Matthew Beck, Michael Millhollen
On Thursday, April 23rd, XenoTech participated in “bring your child to work day”. At XenoTech, we are passionate about what...
Subscribe to our Newsletter
Stay up to date with our news, events and research

Do you have a question or a request for upcoming blog content?
We love to get your feedback