Author: Greg Loewen
Can Interactions Between Therapeutic Proteins and Small Molecule Drugs Be Evaluated In Vitro?
- Biologics & Oligonucleotides
- April 4, 2019
- Greg Loewen, Dr. Maciej Czerwinski
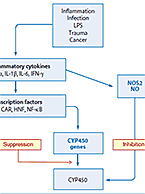
The mechanisms of clearance for small molecule drugs and therapeutic proteins are fundamentally distinct and therefore therapeutic proteins are generally not...
Important Considerations for the Conduct of In Vitro Drug Transporter Assays
- Drug Transporters
- January 11, 2019
- Greg Loewen, Andrea Wolff, Michael Millhollen
Not only can drug transporters affect the absorption and excretion of drugs, they can be involved in pharmacokinetic-based drug-drug interactions...
Coverage from the October 2018 PBSS DDI Workshop
- Drug Transporters
- November 5, 2018
- Greg Loewen, Michael Millhollen
The Pharmaceutical and BioScience Society (PBSS) hosted the workshop “Drug-Drug Interactions: Update on Risk Assessment, Clinical Evaluation and Regulatory Requirements” on October...
AAPS-FDA Drug Transporters
- Regulatory Guidance
- May 30, 2018
- Greg Loewen, Michael Millhollen
The American Association of Pharmaceutical Scientists (AAPS) and U.S. Food and Drug Administration (FDA) co-sponsored the workshop “Drug Transporters in ADME:...
Navigating the Transporter Wave of 2013
- Regulatory Guidance
- August 28, 2013
- Greg Loewen
The International Transporter Consortium (ITC) issued 7 papers in the July 2013 issue of Clinical Pharmacology & Therapeutics. These 7...
Are in vitro drug metabolism and drug-drug interaction studies critical for an IND?
- ADME 101™
- November 1, 2022
- Dr. Andrew G. Taylor, Michael Millhollen, Isis Smith, Amara Millhollen
A Guide to What, Why & When to Conduct ADME Studies When drug developers are preparing their IND, drug metabolism...
Spotlight on Efflux and Uptake Drug Transporters in In Vitro Drug-Drug Interaction Studies
- Drug Transporters
- June 23, 2022
- Dr. Andrew G. Taylor, Michael Millhollen, Isis Smith
What are drug transporters? Drug transporters are membrane-bound proteins that assist in the movement of drugs into or out of...
Which Hepatocytes Should I Use for What Studies?
- Test Systems & Methods
- March 25, 2022
- Dr. Chris Bohl, Michael Millhollen, Isis Smith
Primary hepatocytes are considered the gold standard for ADME/DMPK studies because they are the most representative in vitro test system. However, not all hepatocyte formats…
Timing of In Vitro Studies: Early, Thorough ADME for Your Compound’s Success
- Regulatory Guidance
- February 13, 2020
- Madison Esely-Kohlman, Dr. Chris Bohl, Greg Loewen
Beyond the need for evidence that a drug works, Food and Drug Administration (FDA) and other regulatory bodies around the...
How Can I Make Sure My Data Meets Regulatory Expectations?
- Regulatory Guidance
- January 10, 2020
- Madison Esely-Kohlman, Greg Loewen
Regulatory authorities publish updated guidance documents that share their expectations for endpoints and test systems with drug developers, but sometimes it is difficult to...
What In Vitro Metabolism and DDI Studies Do I Actually Need?
- Regulatory Guidance
- November 25, 2019
- Madison Esely-Kohlman, Greg Loewen
Though there is no ‘roadmap’ spelling out required studies to achieve regulatory approval for clinical entry, a drug candidate’s metabolism...
Subscribe to our Newsletter
Stay up to date with our news, events and research

Do you have a question or a request for upcoming blog content?
We love to get your feedback