Year: 2019
Official PMDA English Translation
- Regulatory Guidance
- May 7, 2019
- Madison Esely-Kohlman, Dr. Brian Ogilvie
The Japanese regulatory agent PMDA (Pharmaceuticals and Medical Devices Agency) recently published an official English translation of their final Drug Interaction Guideline...
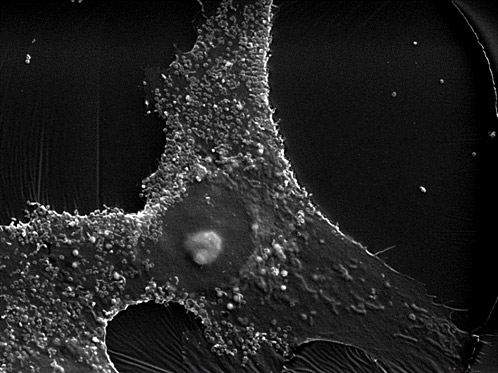
Quality Control is What Makes the Comprehensive Collection of Cell Lines from JCRB Among the Highest Regarded and Most Widely Distributed in the World
- Test Systems & Methods
- April 23, 2019
- Madison Esely-Kohlman, Dr. Arihiro Kohara, Kimiho Yamada
About JCRB cell bank In addition to focus on preclinical ADME in vitro testing services and complementary products, XenoTech offers to our clients...
Minding Your Binding: Plasma Protein Binding Potential Study Now Available at Our US Labs
- Test Systems & Methods
- April 15, 2019
- Madison Esely-Kohlman, Andrea Wolff, Dr. Joanna Barbara
While liver and intestine are important in ADME, behavior in blood is also crucial. When an orally administered drug or...
Studies in Japan: Easier & More Beneficial than You Might Think
- In Vivo & Radiolabeling
- April 8, 2019
- Madison Esely-Kohlman
XenoTech is well-known to pharmaceutical companies and toxicology academics alike for unparalleled experience in quality in vitro ADME/DMPK/DDI studies and complementary products,...
Can Interactions Between Therapeutic Proteins and Small Molecule Drugs Be Evaluated In Vitro?
- Biologics & Oligonucleotides
- April 4, 2019
- Greg Loewen, Dr. Maciej Czerwinski
The mechanisms of clearance for small molecule drugs and therapeutic proteins are fundamentally distinct and therefore therapeutic proteins are generally not...
New Liver Tissue Microarrays Available – What Would Benefit Your Research?
- Disease-Specific Resources
- April 2, 2019
- Madison Esely-Kohlman, Dr. Maciej Czerwinski
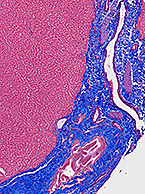
As technology evolves to better meet needs in exploration of drug metabolism pathways and disease mechanisms, familiarization with appropriate and...
Disease-State Test Systems
- Test Systems & Methods
- January 13, 2019
- Michael Millhollen, Dr. Chris Bohl
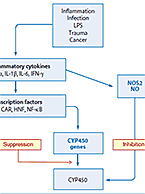
XenoTech has been working with and supplying reagents for the pre-clinical ADME field for well over two decades. Years of...
Important Considerations for the Conduct of In Vitro Drug Transporter Assays
- Drug Transporters
- January 11, 2019
- Greg Loewen, Andrea Wolff, Michael Millhollen
Not only can drug transporters affect the absorption and excretion of drugs, they can be involved in pharmacokinetic-based drug-drug interactions...
5 Keys to Set Your Contract Research Organization (CRO) Collaboration Up for Success
- Updated Offerings
- January 7, 2019
- Michael Millhollen, Kelsey Acree
Over the past 25 years of performing ADME/DMPK/DDI contract research, we’ve learned some key strategies to maximize the efficiency and effectiveness...
Subscribe to our Newsletter
Stay up to date with our news, events and research

Do you have a question or a request for upcoming blog content?
We love to get your feedback