Tox
Reactive Metabolite Detection Studies: Cysteine Trapping
The Role of In Vitro Assays in Selecting the Right Species for Your Small Molecule Program
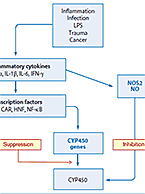
In Vitro Cholestatic DILI & Mitochondrial Toxicity Studies to Assess Hepatotoxicity
Toxicokinetic (TK) Analysis for Preclinical Drug Development
The main goal of preclinical toxicokinetic (TK) studies is to establish a correlation between a candidate compound’s concentration or dose...
Toxicity evaluation by high content analysis of 3D cultured primary human hepatocytes
What is DMPK and how does it fit into drug development?
Drug metabolism and pharmacokinetics (DMPK) is a core discipline in drug development that considers the biotransformation of a drug compound...
Meet the Scientist: Pallavi Limaye
We have welcomed a new consultant to the team! Dr. Pallavi Limaye now serves as a Director in the Scientific...
ADME and Drug-Drug Interactions for the Toxicologist
Highlights from the recent webinar presented by our newest expert consultant, Dr. Pallavi Limaye In her recent webinar (now available for...
In Vitro Evaluation of Drug-Drug Interaction (DDI) Potential
In its most recent in vitro drug interaction guidance update, the US Food and Drug Administration (FDA) emphasized harmony with the...
Four ways to optimize preclinical in vitro data to mitigate risk of late-stage clinical failure
1. Collect high-quality data to make informed, confident go/no go decisions for moving your drug candidate forward If you need...
How can in vitro and in vivo studies help me understand my drug’s clearance?
Systemic clearance, denoting how much drug is cleared from a volume of blood per unit of time, is of critical...
What You Need to Know About Micro-Autoradiography (mARG) Distribution Studies
In vivo determination of drug localization in tissue can be uniquely informative to drug developers investigating distribution within the context of...
What In Vitro Metabolism and DDI Studies Do I Actually Need?
Though there is no ‘roadmap’ spelling out required studies to achieve regulatory approval for clinical entry, a drug candidate’s metabolism...
Mass Balance Studies: What You Need and Why You Need It
In vivo mass balance studies are an important element of nonclinical drug development, to inform first in-human (FIH) studies and to...
Why You Need QWBA for Human Radiolabeled ADME Studies
A quantitative whole body autoradiography (QWBA) study provides data required for Human Radiolabeled ADME Studies1. Quantitative whole body autoradiography (QWBA) is an in...
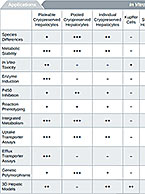
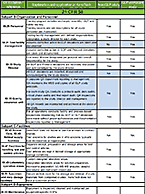
How to Choose the Right Test Systems for Your DMPK Studies
Test systems for DMPK in vitro studies are part of the very foundation of our company. Our labs were borne of...
Human mAb stability and cytotoxicity in rat hepatocytes with a surrogate peptide approach
Human monoclonal antibody stability in rat cell cultures using surrogate peptide LC-MS/MS
In Vitro Induction Studies: Elements of Design and Important Considerations in Data Analysis
Why do induction studies? Induction potential is an important piece of the drug-drug interaction (DDI) component of an IND submission. Simply put, we...
Minding Your Binding: Plasma Protein Binding Potential Study Now Available at Our US Labs
While liver and intestine are important in ADME, behavior in blood is also crucial. When an orally administered drug or...
Studies in Japan: Easier & More Beneficial than You Might Think
XenoTech is well-known to pharmaceutical companies and toxicology academics alike for unparalleled experience in quality in vitro ADME/DMPK/DDI studies and complementary products,...
Can Interactions Between Therapeutic Proteins and Small Molecule Drugs Be Evaluated In Vitro?
The mechanisms of clearance for small molecule drugs and therapeutic proteins are fundamentally distinct and therefore therapeutic proteins are generally not...
Important Considerations for the Conduct of In Vitro Drug Transporter Assays
Not only can drug transporters affect the absorption and excretion of drugs, they can be involved in pharmacokinetic-based drug-drug interactions...
To GLP or not to GLP?
That is the question. . . Knowing the answer may save you time and money Good Laboratory Practices (GLP) are...
Choosing a Relevant Small Animal Model for Pharmacokinetic or Toxicity Studies
Choosing the most suitable small animal model is crucial for pharmacokinetic and/or toxicology studies in order to get usable, relevant data. Due to potential...