

October Presentations on In Vitro Effects of Biologics on CYP Enzymes and Regulatory DDI Guidances
- Regulatory Guidance
- October 26, 2018
- Michael Millhollen
On Oct. 15th at the Peptide ADME Discussion Group Workshop in Gothenburg, Sweden, Dr. Brian Ogilvie presented on In vitro Direct and Cytokine-Mediated Effects of Therapeutic Peptides and other Biologics on CYP Enzymes. If you could not attend his presentation, he is planning on co-presenting a webinar on the topic with Dr. Maciej Czerwinski in early 2019. Please submit a webinar request form to be notified as details become available.
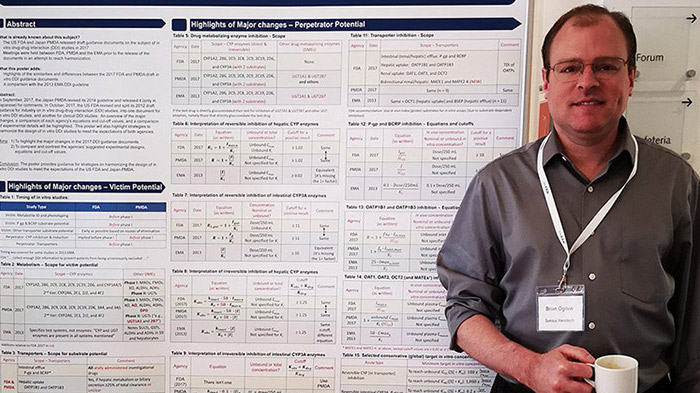
On Oct. 18th at the Drug Metabolism Discussion Group (DMDG) – Swedish Academy of Pharmaceutical Sciences (SPS) Joint Meeting in Gothenburg, Sweden, Dr. Brian Ogilvie presented on the Comparison Between the US FDA, Europe EMA and Japan PMDA In Vitro DDI Guidances: Are we Closer to Harmonization? Since Brian has presented on this topic at numerous events, you can use the links below to access a recording of his presentations and download the slides, or access his incredibly useful reference comparison poster:
Watch “Essential Considerations on the New FDA In Vitro DDI Guidance (the What, the Why, and the Wow)”
Download our poster comparing the FDA, EMA & PMDA DDI guidance documents
Listen to Drs. Ogilvie and Parkinson discuss a comparison of the FDA, EMA & PMDA DDI guidances
Learn more about XenoTech’s expertise and capabilities
Contact us with any questions
Learn more about:
About the Authors
Related Posts
Subscribe to our Newsletter
Stay up to date with our news, events and research

Do you have a question or a request for upcoming blog content?
We love to get your feedback
